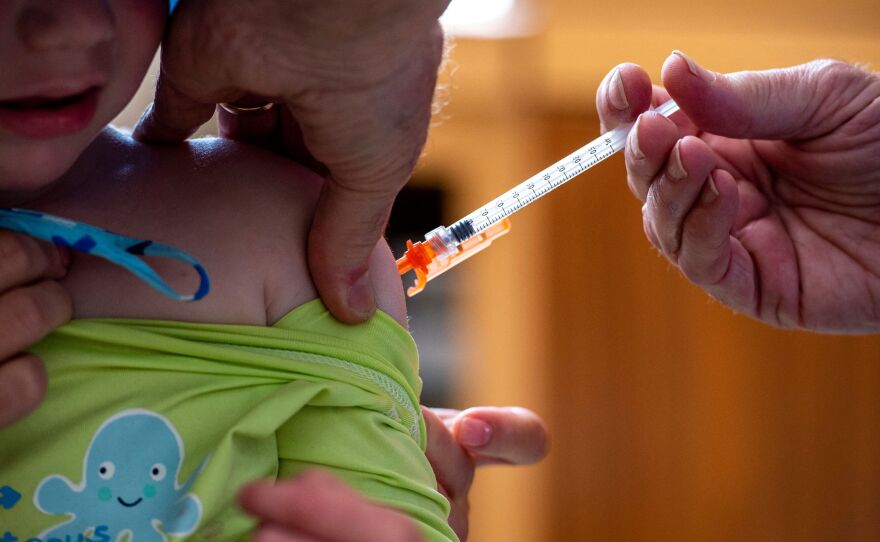
The Covid-19 vaccine will no longer be included in the recommended vaccines for healthy children and expectant women on the immunization schedule of the US Centers for Disease Control and Prevention, as announced by US Health and Human Services Secretary Robert F. Kennedy Jr. on Tuesday.
As of mid-Tuesday, the immunization schedule that was published online had not been altered.
In a video that was uploaded to social media, Kennedy disclosed the unconventional action. Dr. Jay Bhattacharya, Director of the National Institutes of Health, and Dr. Marty Makary, Commissioner of the US Food and Drug Administration, flanked him.
Kennedy stated that the Covid vaccine has been delisted from the CDC’s recommended immunization schedule for healthy children and expectant women as of today. “Despite the absence of any clinical data to substantiate the repeat booster strategy in children, the Biden administration urged healthy children to receive yet another Covid shot last year.”
Kennedy did not provide any scientific evidence to support the modification of the recommendations.
READ MORE: Robert F. Kennedy Jr. Will Not Be Charged With Dumping A Dead Bear
However, experts warn that the transition will have catastrophic repercussions, particularly for expectant women and their infants. Both are regarded as having a heightened risk of experiencing severe complications as a result of Covid-19 infections. Research has demonstrated that the risk of hospitalization for expectant women and infants under the age of six months is diminished by the Covid-19 vaccination.

“This is in direct opposition to the safety and benefits of Covid-19 vaccination for both pregnant women and young children,” stated an individual who is privy to the CDC’s vaccine recommendations. The individual requested anonymity due to concerns regarding potential retribution.
In a statement issued on Tuesday, Dr. Steven Fleischman, the president of the American College of Obstetricians and Gynecologists, stated that “the science has not changed.” It is evident that COVID infection during pregnancy can have catastrophic consequences, including significant disability and devastating consequences for families. The COVID vaccine is secure for pregnant women, and it can safeguard the health of our patients and their infants.
Fleischman went on to explain that the majority of hospitalized infants under the age of six months, who are not yet eligible for vaccination, are born to unvaccinated mothers. This is supported by an increasing body of evidence that demonstrates the extent to which vaccination during pregnancy protects the infant after birth.

According to specialists, Kennedy’s decision may influence the government’s procurement of the vaccines and insurance coverage.
Dr. Paul Offit, the director of the Vaccine Education Center at Children’s Hospital of Philadelphia and a pediatrician, stated that the Covid-19 vaccines will become less accessible and affordable as a result of this.
The announcement is in stark contrast to Kennedy’s statements since assuming the position of HHS secretary. “I have consistently maintained throughout my campaign, and in every public statement I have made, that I will not confiscate people’s vaccines,” he stated to CBS in April. “I will ensure that we have high-quality science to enable individuals to make well-informed decisions.”
However, Offit stated on Tuesday, “He is lying, as that is precisely what he is doing.” He is removing them.
A typical process is circumvented by change.
Kennedy circumvented the government’s customary process for evaluating and recommending vaccines for Americans by disclosing the change.

The FDA typically assesses the safety and efficacy of vaccines by examining data from clinical trials and other research. The Advisory Committee on Immunization Practices (ACIP), a committee of independent vaccine experts, and the CDC will recommend the use of a vaccine, including the frequency of administration and the individuals who should receive it, after the FDA has approved it. The CDC is not obligated to comply with ACIP’s recommendations; however, it frequently does.
The regulatory implications of ACIP’s recommendations are substantial. Insurance companies are obligated to provide coverage for adult vaccines that have been recommended by the ACIP under the Affordable Care Act. The advisory committee also determines whether vaccines should be included in the federal Vaccines for Children program, which offers vaccines to children who would not otherwise be able to afford them.
The CDC’s decision to revoke its recommendations for pregnant women and healthy children has prompted inquiries regarding the coverage of Covid-19 vaccines by insurance providers and the government’s procurement of these vaccines.
Health insurance companies have the discretion to provide coverage for Covid-19 vaccinations independently, even in the absence of a formal CDC recommendation. It is probable that they will do so, as scientific evidence supports the vaccines’ safety and efficacy, according to an individual who is acquainted with the legal implications of disregarding the CDC’s recommendation. The individual requested anonymity due to concerns about retribution.
The individual stated that it is possible that the Vaccines for Children program will not provide coverage for the vaccinations.

The Infectious Diseases Society of America, a professional organization, stated that the decision could impede the ability of millions of Americans to obtain vaccines.
In a statement, Dr. Tina Tan, the president of IDSA, emphasized the importance of insurers maintaining coverage for COVID-19 vaccines to ensure that all Americans are able to make the best decisions to safeguard themselves and their families from severe illness, hospitalization, and death. “IDSA also encourages Congress to implement meaningful and essential oversight to guarantee that the Department of Health and Human Services’ decision-making processes are appropriate, as this will affect individuals of all ages.”
Kennedy’s announcement could potentially be challenged in court, according to other experts.
“This is an extremely poor administrative procedure,” wrote Dorit Reiss, a law professor at the University of California at San Francisco, in a social media post.
“In order to prevent being deemed arbitrary and capricious under administrative law, an agency’s decision must satisfy specific criteria, such as the agency’s fact-finding process and the connection between the facts and the decisions,” Reiss wrote. “A one-minute video on Twitter does not quite achieve the desired outcome.”

Additional modifications to the Covid-19 vaccine
The CDC’s vaccine advisers had already been contemplating a modification to the nation’s Covid-19 vaccine recommendations. The committee was contemplating the possibility of issuing more specific risk-based recommendations, which would encompass individuals aged 65 and older and those with compromised immune function, rather than the general recommendation that all individuals aged 6 months and older receive an updated annual vaccination.
The majority of the working group members who were analyzing the change in April expressed their support for narrowing the recommendation during a meeting. The complete committee intended to vote on it at their upcoming meeting in June.
Rather, Makary and Dr. Vinay Prasad, director of the FDA’s Center for Biologics Evaluation and Research, published an editorial in the New England Journal of Medicine to announce a modification to the FDA’s approval process for Covid-19 vaccines. The agency stated that it would require placebo-controlled clinical trials before it would approve new vaccines for healthy children and adults aged 64 and younger.
“The benefit of repeat dosing, particularly among low-risk individuals who may have previously received multiple doses of Covid-19 vaccines, had multiple Covid-19 infections, or both, is uncertain,” Makary and Prasad wrote, despite the significant scientific, medical, and regulatory accomplishment of the rapid development of multiple Covid-19 vaccines in 2020.”
They claimed that the new policy would encourage drug companies to produce supplementary evidence to demonstrate that the injections are beneficial for healthy young adults and children.
Dr. Peter Hotez, director of vaccine development at Texas Children’s Hospital, stated that the action appears to be inconsistent with the principles of the health freedom movement that Kennedy embodies.
“It deprives parents and their pediatricians of the ability to make decisions, and instead, it is now made by the government, which is the antithesis of the health freedom that MAHA advocates for,” stated Hotez, referring to the acronym for Make America Healthy Again.

Considering the decision to vaccinate children
According to experts, the evidence regarding the benefits of Covid-19 vaccination for expectant women is unambiguous; however, the situation is more complex for children due to the scarcity of studies conducted in this age group.
The CDC’s vaccine advisory committee heard evidence at its most recent meeting in April that children accounted for approximately 4% of all Covid-19 hospitalizations during the respiratory virus season of the previous fall and winter.
The youngest age groups are at the highest risk of Covid-19 hospitalizations, with infants under 6 months of age being particularly susceptible. Nevertheless, the risk can be mitigated through maternal vaccination.
The committee also reviewed evidence regarding the risk of myocarditis, or cardiac inflammation, which is exceedingly uncommon in conjunction with Covid-19 vaccination. Myocarditis rates have steadily decreased, and there were only five verified cases in individuals aged 12 to 40 within 21 days of receiving a Pfizer Covid-19 vaccine during the previous autumn and winter. Nevertheless, the researchers observed the same number of cases in a comparison group, which led them to the conclusion that the Covid-19 vaccines did not increase the risk of myocarditis during the previous season.
Nevertheless, the FDA has mandated that Pfizer and Moderna, the manufacturers of Covid-19 vaccines, amend their warning labels to include additional information regarding the risk of myocarditis.
Step into the ultimate entertainment experience with Radiant TV! Movies, TV series, exclusive interviews, live events, music, and more—stream anytime, anywhere. Download now on various devices including iPhone, Android, smart TVs, Apple TV, Fire Stick, and more!